Cathodic protection (CP) is a method of corrosion control that uses an external anode as a current source to impress direct current (DC) through the soil onto a metallic structure or pipeline. CP mitigates the flow of corrosion currents that occur when a pipeline is installed in corrosive soil. The pipeline is made more electronegative with respect to the soil, and the pipeline becomes the cathode in the corrosion cell. The current flows along the pipeline to the drain wire to complete the circuit.
Let’s take it back to the basics…
Three conditions must be present to form a corrosion cell:
Electrical continuity
Electrolytic pathway
Potential difference
For a pipe installed in soil, potential differences exist on the pipe’s surface due to soil nonconformities and different concentrations of alloying elements within the microstructure (for example, in carbon steel, the percentage of carbon varies at different parts of the microstructure). This sets up tiny corrosion cells all over the surface of the pipe. In order to inhibit those corrosion cells, one of the three conditions listed above has to be removed.
Condition examples include:
The pipe is electrically continuous with itself, so the electrical continuity cannot be removed.
The soil around the pipe provides the electrolytic pathway. A perfect coating could provide a barrier and disrupt the electrolytic pathway; however, no coating is perfect. Coating imperfections called holidays exist, making the pipe susceptible to corrosion at those locations.
Therefore, one option remains. Remove the potential differences on the surface of the pipe, and that is exactly what CP does.
How Does CP Mitigate Potential Differences on the Pipe?
In layman’s terms, CP is a controlled and intentional corrosion cell. CP sets up a large corrosion cell where the pipe is the cathode (more electropositive), and a sacrificial component is the anode (more electronegative). This large, intentional corrosion cell overcomes the small, natural corrosion cells that would otherwise be present on the pipe surface using the following logic:
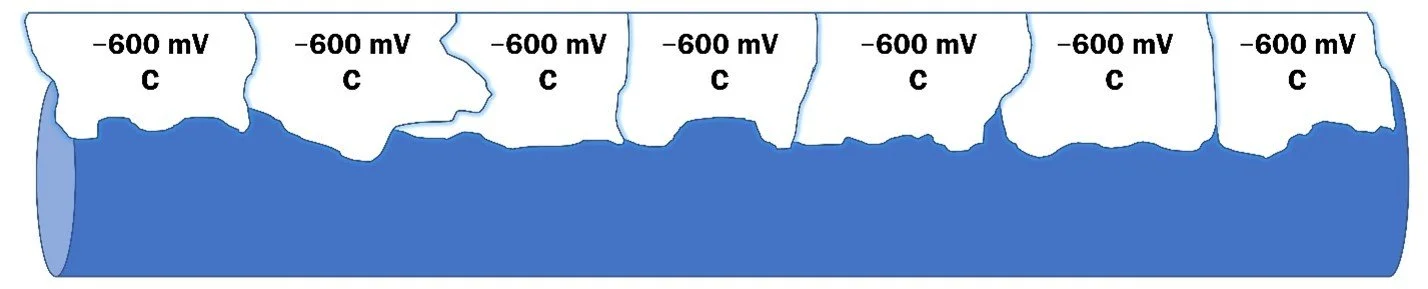
1.) On an unprotected pipe surface, there is a range of potentials present, which allow corrosion cells to form.
2.) As CP current is applied to the pipe, the most electropositive locations become more electronegative, disrupting some corrosion cells.
3.) The CP current is increased until the pipe has equal potentials across the surface. At this point, the pipeline is considered cathodically protected and external corrosion if effectively mitigated.
At V&A, we are passionate about designing corrosion prevention and response for metal and concrete pipelines as a critical infrastructure preservation best practice. We welcome your CP System and Corrosion questions in the comment section below.
More on Cathodic Protection from V&A:
4 Key Factors in Estimating Cathodic Protection System Costs in Your Pipeline Project